Chapter 1 - Standard Infection Control Precautions (SICPs)
Chapter 1 - Standard Infection Control Precautions (SICPs)
 Standard Infection Control Precautions (SICPs), covered in this chapter are to be used by all staff, in all care settings, at all times, for all patients1 whether infection is known to be present or not to ensure the safety of those being cared for, staff and visitors in the care environment.
Standard Infection Control Precautions (SICPs), covered in this chapter are to be used by all staff, in all care settings, at all times, for all patients1 whether infection is known to be present or not to ensure the safety of those being cared for, staff and visitors in the care environment.
The Hierarchy of Controls should also be considered in controlling exposures to occupational hazards which include infection risks.
SICPs are the basic infection prevention and control measures necessary to reduce the risk of transmission of infectious agent from both recognised and unrecognised sources of infection.
Sources of (potential) infection include blood and other body fluids secretions or excretions (excluding sweat), non-intact skin or mucous membranes, any equipment or items in the care environment that could have become contaminated and even the environment itself if not cleaned and maintained appropriately.
The application of SICPs during care delivery is determined by an assessment of risk to and from individuals and includes the task, level of interaction and/or the anticipated level of exposure to blood and/or other body fluids.
To be effective in protecting against infection risks, SICPs must be applied continuously by all staff. The application of SICPs during care delivery must take account of:
- risk to and from the individual for whom care is being provided
- the task to be undertaken
- level of interaction
- the anticipated level of exposure to blood and/or other body
Doing so allows staff to safely apply each of the 10 SICPs by ensuring effective infection prevention and control is maintained.
SICPs implementation monitoring must also be ongoing to demonstrate safe practices and commitment to patient, staff and visitor safety.
Further information on using SICPs for Care at Home can be found on the NHS National Education Scotland (NES) website.
1The use of the word 'Persons' can be used instead of 'Patient' when using this document in non-healthcare settings.
Last updated: 28 August 2023
1.1 Patient Placement/Assessment for infection risk
Patients must be promptly assessed for infection risk on arrival at the care area (if possible, prior to accepting a patient from another care area) and should be continuously reviewed throughout their stay. This assessment should influence patient placement decisions in accordance with clinical/care need(s).
Patients who may present a particular cross-infection risk should be isolated on arrival and appropriate clinical samples and screening undertaken as per national protocols to establish the causative pathogen. This includes but is not limited to patients:
- With symptoms such as loose stools or diarrhoea, vomiting, fever or respiratory symptoms.
- With a known (laboratory confirmed) or suspected infectious pathogen for which appropriate duration of precautions as outlined in A-Z pathogens are not yet complete.
- Known or suspected to have been previously positive with a
Multi-drug Resistant Organism (MDRO), for example MRSA, CPE. - Who have been a close contact of a person who has been colonised or infected with CPE in the last 12 months.
- Who have been hospitalised outside Scotland in the last 12 months (including those who received dialysis).
Resources
For assessment of infection risk see Section 2: Transmission Based Precautions.
Further information can be found in the patient placement literature review.
Further information regarding general respiratory screening questions can be found within the resources section of the NIPCM.
1.2 Hand Hygiene
Please note that the term ‘alcohol-based hand rub (ABHR)’ has now been updated to ‘hand rub’. A hand rub (alcohol or non-alcohol based) can be used if it meets the required standards. Please see further information in the hand hygiene products literature review.
Hand hygiene is considered an important practice in reducing the transmission of infectious agents which cause infections.
Adherence with the following points is essential to ensure effective hand hygiene:
- expose forearms (bare below the elbows)
- remove all hand/wrist jewellery* including any embedded jewellery (a single, plain metal finger ring or ring dosimeter (radiation ring) is permitted but should be removed (or manipulated) during hand hygiene). Bracelets or bangles such as the Kara which are worn for religious reasons should be able to be pushed higher up the arm and secured in place to enable effective hand hygiene which includes the wrists
- ensure fingernails are clean, short and that artificial nails or nail products are not worn
- cover all cuts or abrasions with a waterproof dressing
Hand washing should be extended to the forearms if there has been exposure of forearms to blood and/or body fluids.
Hand washing sinks must only be used for hand hygiene and must not be used for the disposal of other liquids. See Chapter 4 - 4.1.4 Management of water outlets including taps and showers).
*Scottish Ambulance Service (SAS) staff should follow this guidance in conjunction with The Association of Ambulance Chief Executives (AACE) position statement: BBE-position-statement-March-2025-V3.0.pdf
To perform hand hygiene
Hand rubs must be available for staff as near to point of care as possible. Where this is not practical, personal hand rub dispensers should be used.
Application of sufficient volume of hand rub to cover all surfaces of the hands is important to ensure effective hand hygiene. Manufacturer’s instruction should be followed for the volume of hand rub required to provide adequate coverage for the hands. In the absence of manufacturers instructions, volumes of approximately 3ml are recommended to ensure full coverage.
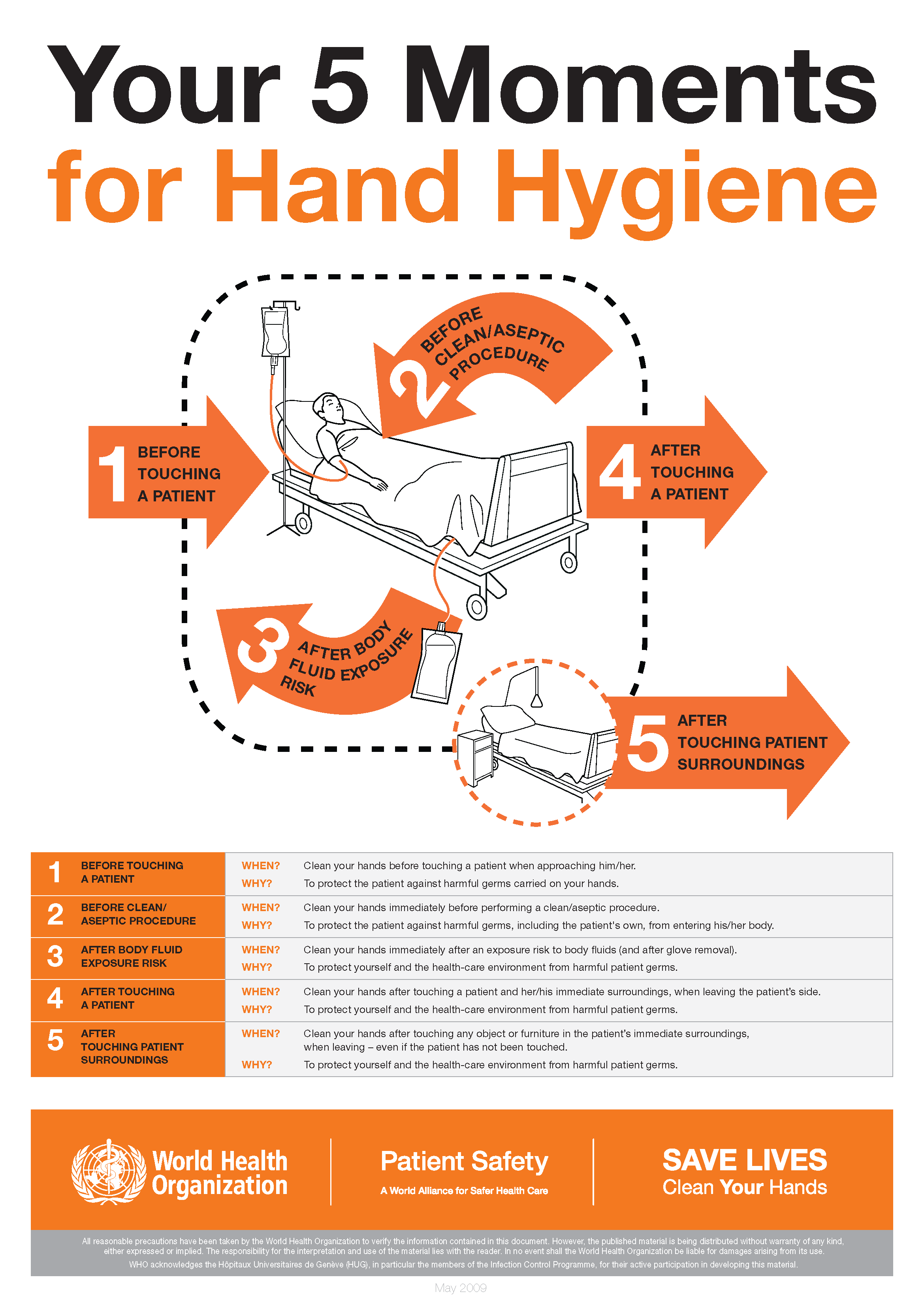
The World Health Organization’s ‘5 moments for hand hygiene’ should be used to highlight the key indications for hand hygiene.
- before touching a patient
- before clean/aseptic procedures. If hand rub cannot be used, then antimicrobial liquid soap should be used
- after body fluid exposure risk
- after touching a patient
- after touching a patient’s immediate surroundings
Some additional examples of hand hygiene moments include but are not limited to:
- before handling medication
- before preparing food
- before donning (putting on) and after doffing (taking off) PPE
- after visiting the toilet
- between carrying out different care activities on the same patient
- after cleaning and disinfection procedures
- after handling waste
Download and print the 5 moments of hand hygiene poster.
Wash hands with non-antimicrobial liquid soap and water if:
- hands are visibly soiled or dirty
- hands are potentially contaminated with blood, other body fluids or excretions
- caring for patients with vomiting or diarrhoeal illnesses
- caring for a patient with a suspected or known gastro-intestinal infection, for example Norovirus or a spore forming organism such as Clostridioides difficile
Hands should be washed with warm/tepid water to mitigate the risk of dermatitis associated with repeated exposures to hot water and to maximise hand washing compliance. Compliance may be compromised where water is too hot or too cold. Hands should be dried thoroughly following hand washing using a soft, absorbent, disposable paper towel from a dispenser which is located close to the sink but beyond the risk of splash contamination.
In all other circumstances use hand rub for routine hand hygiene during care.
Staff working in the community should carry a supply of hand rub to enable them to perform hand hygiene at the appropriate times.
Where staff are required to wash their hands in the service user’s own home they should do so for at least 20 seconds using any hand soap available.
Staff should carry a supply of disposable paper towels for hand drying rather than using hand towels in the individual’s own home. Once hands have been thoroughly dried, hand rub should be used.
The use of antimicrobial hand wipes is only permitted where there is no access to running water. Staff must perform hand hygiene using hand rub immediately after using the hand wipes and perform hand hygiene with soap and water at the first available opportunity.
Resources
(The video above demonstrating Hand Washing and Drying Technique was produced by NHS Ayrshire and Arran)
For how to:
- wash hands see Appendix 1
- hand rub see Appendix 2
Hand hygiene posters and leaflets can be found at Wash Your Hands of Them Resources.
WHO World Hand Hygiene Day 5 May 2025 - It might be gloves. It's always hand hygiene resources are available.
Skin care
- Hand rubs when used for hand hygiene should contain emollients in their formulation.
- Warm/tepid water should be used to reduce the risk of dermatitis. Hot water should be avoided.
- Pat hands dry thoroughly after hand washing using disposable paper towels. Avoid rubbing which may lead to skin irritation/damage.
- Use an emollient hand cream during work and when off duty. These should be applied all over the hands including between the fingers and the back of the hands.
- Do not use refillable dispensers or provide communal tubs of hand cream in the care setting.
- Staff with skin problems should seek advice from occupational health or their GP.
- Barrier creams should not be used in the workplace.
Surgical hand antisepsis
Surgical scrubbing/rubbing applies to persons undertaking surgical and some invasive procedures.
Perform surgical scrubbing/rubbing before donning sterile theatre garments or at other times, for example prior to insertion of central vascular access devices.
Surgical scrubbing using an antimicrobial surgical scrub product should be used for the first surgical hand antisepsis of the day. Or perform hand hygiene using water and a non-antimicrobial liquid soap prior to the first surgical antisepsis of the day, this can be carried out in an adjacent clinical area.
For surgical scrubbing
- Remove all hand/wrist jewellery.
- Nail brushes should not be used for surgical hand antisepsis.
- Nail picks (single-use) can be used if nails are visibly dirty.
- Soft, non-abrasive, sterile (single-use) sponges may be used to apply antimicrobial liquid soap to the skin if licensed for this purpose.
- Use an antimicrobial liquid soap licensed for surgical scrubbing or hand rub licensed for surgical rubbing (as specified on the product label).
- Hand rub can be used between surgical procedures if licensed for this use or between glove changes if hands are not visibly soiled.
- Skin should be blotted dry with sterile single-use towels.
Resources
- For surgical scrubbing technique see Appendix 3.
- For surgical rubbing technique see Appendix 4.
Further information can be found in the Hand Hygiene literature reviews:
1.3 Respiratory and Cough Hygiene
 Respiratory and cough hygiene is designed to minimise the risk of cross-transmission of respiratory illness (pathogens).
Respiratory and cough hygiene is designed to minimise the risk of cross-transmission of respiratory illness (pathogens).
- Cover the nose and mouth with a disposable tissue when sneezing, coughing, wiping and blowing the nose. If a disposable tissue is not available use elbow to cover the nose and mouth when coughing or sneezing.
- Patients showing symptoms of respiratory illness should be encouraged to wear a surgical (TYPE II R FRSM) face mask where it is clinically safe and tolerated by the wearer.
- Dispose of used tissues and face masks promptly into a waste bin.
- In the absence of disposable tissues and hand hygiene facilities only, individuals should cough or sneeze into their elbow/sleeve.
- Wash hands with non-antimicrobial liquid soap and warm water after coughing, sneezing, using tissues, or after contact with respiratory secretions or objects contaminated by these secretions.
- Where there is no running water available or hand hygiene facilities are lacking, staff may use hand wipes followed by ABHR and should wash their hands at the first available opportunity.
- Keep contaminated hands away from the eyes nose and mouth.
Staff should promote respiratory and cough hygiene helping those who need assistance with this, for example elderly and children, providing patients with tissues, plastic bags for used tissues and hand hygiene facilities as necessary.
Resources
Further information can be found in the cough etiquette/respiratory hygiene literature review.
1.4 Personal Protective Equipment
 Before undertaking any care task or procedure staff should assess any likely exposure to blood and/or body fluids and ensure PPE is worn that provides adequate protection against the risks associated with the procedure or task being undertaken.
Before undertaking any care task or procedure staff should assess any likely exposure to blood and/or body fluids and ensure PPE is worn that provides adequate protection against the risks associated with the procedure or task being undertaken.
All PPE should be:
- located close to the point of use
- stored to prevent contamination in a clean/dry area until required for use (expiry dates must be adhered to)
- single-use only items unless specified by the manufacturer, for example reusable items such as non-disposable goggles, face shields or visors
- changed immediately after each patient and/or following completion of a procedure or task
- disposed of after use into the correct healthcare waste stream
Reusable PPE
Reusable PPE items, for example non-disposable goggles, face shields or visors must be cleaned/decontaminated once removed or placed within a designated container for subsequent cleaning/decontamination with decontamination schedules in place and responsibility assigned.
Reusable PPE must be cleaned/decontaminated as per manufacturers instructions or in line with local policies or procedures.
Resources
Further information on best practice for PPE use for SICPs can be found in Appendix 15.
Gloves must:
- be worn when exposure to blood, body fluids, (including but not limited to secretions and/or excretions), non-intact skin, lesions and/or vesicles, mucous membranes, hazardous drugs and chemicals, for example cleaning agents is anticipated/likely. (Scottish National Blood Transfusion Service (SNBTS) adopt practices that differ from those stated in the National Infection Prevention and Control Manual)
- Gloves are a single-use item and should be donned immediately prior to exposure risk and should be changed immediately after each use or upon completion of a task;
- never be worn inappropriately in situations such as to go between patients, move around a care area, work at IT workstations
- be changed if a perforation or puncture is suspected or identified
- be appropriate for use, fit for purpose and well-fitting
- not be worn as a substitute to hand hygiene.
Double gloving is only recommended during some Exposure Prone Procedures (EPPs), for example orthopaedic and gynaecological operations or when attending major trauma incidents and when caring for a patient with a suspected or known High Consequence Infectious disease. Double gloving is not necessary at any other time.
Resources
For appropriate glove use and selection see Appendix 5.
Further information can be found in the Gloves literature review.
Aprons must be:
- worn to protect uniform or clothes when contamination is anticipated/likely
- worn when in direct care contact with a patient or their immediate environment, for example providing toileting support or changing bed linen
- changed between patients and following completion of a procedure or task
Full body gowns/fluid repellent coveralls must be:
- worn when there is a risk of extensive splashing of blood and/or other body fluids, for example in the operating theatre
- worn when a disposable apron provides inadequate cover for the procedure/task being performed
- changed between patients and immediately after completion of a procedure or task
The choice of apron or gown is based on a risk assessment and anticipated level of body fluid exposure. Routine sessional use of gowns/aprons is not permitted.
Sterile surgical gowns must be:
- worn by all scrubbed members of the operating theatre surgical team
- worn for insertion of central venous catheters, insertion of peripherally inserted central catheters, insertion of pulmonary artery catheters and spinal, epidural and caudal procedures
Reusable gowns must:
- not be worn in the operating theatre environment or for aseptic surgical procedures
- be appropriately processed between uses based on manufacturer’s instructions
If hand hygiene with soap and water is required, this should not be performed whilst wearing an apron/gown in line with a risk of apron/gown contamination; hand hygiene using ABHR is acceptable.
Resources
Further information can be found in the Aprons/Gowns literature review.
Eye/face protection must:
- be worn when there is an anticipated risk of splashing or spraying of blood or bodily fluids and always during Aerosol Generating Procedures
- be compatible with other items of PPE and worn in accordance with manufacturer’s instructions
- not be touched when worn or worn around the neck or on top of the head when not in use
- provide adequate protection from the risks of the task being undertaken
- be removed or changed when vision is impaired due to visible soiling or contamination or damage, when task complete and exposure risk has ended or immediately before leaving the work area
Prescription eyeglasses and contact lenses should not be considered a form of eye or face protection
Resources
Further information can be found in the eye/face protection literature review.
Fluid Resistant Type IIR surgical face masks must be:
- worn by a patient known or suspected to be infected with a micro-organism spread by the droplet or airborne route when leaving their room or when moving between clinical areas including transfers by portering staff and ambulance services
- worn if splashing or spraying of blood, body fluids, secretions or excretions onto the respiratory mucosa (nose and mouth) is anticipated/likely. (As part of SICPs a full-face visor may be used as an alternative to fluid resistant Type IIR surgical face masks to protect against splash or spray)
- worn in combination with a full-face shield, integrated half face shield or goggles for AGPs on non-infectious patients
- worn to protect patients from the operator as a source of infection when performing invasive spinal procedures such as myelography, lumbar puncture and spinal anaesthesia, inserting a Central Vascular Catheter (CVC), performing intra-articular (joint) injections
- worn by all scrubbed members of the theatre surgical team for all surgical procedures
- worn by non-scrubbed members of the theatre surgical team if deemed necessary following a risk assessment of exposure to blood and/or body fluids
- well fitting and fit for purpose (fully covering the mouth and nose)
- removed or changed:
- at the end of a procedure/task
- if the integrity of the mask is breached, e.g. from moisture build-up after prolonged use or from gross contamination with blood or body fluids
- in accordance with specific manufacturers’ instructions
Transparent face masks
Transparent face masks may be used to aide communication with patients in some settings.
Transparent face masks must:
- meet the specification standards of the Transparent face mask technical specification
and
- have been approved by the UK Transparent Mask review group for use within health and social care settings
- only be worn in areas where Fluid Resistant Type IIR surgical face masks are used as personal protective equipment.
Resources
Further information can be found in:
- aerosol generating procedures literature review
- surgical face masks literature review
- section 2.4 of the NIPCM
- appendix 11 of the NIPCM
Footwear must be:
- non-slip, impervious, clean and well maintained, and support and cover the entire foot to avoid contamination with blood or other body fluids or potential injury from sharps
- removed before leaving a care area where dedicated footwear is used, for example theatre. Employees must clean and decontaminate footwear upon removal and when visibly soiled with blood and/or body fluids following manufacturers recommended instructions for cleaning and disinfection
- dedicated for use in settings such as theatres and stored in a designated area when not in use
Footwear found to be defective should be repaired or replaced before further use.
Overshoes/shoe covers should not be used in the general health and care environment.
Resources
Further information can be found in the footwear literature review.
Headwear must be:
- worn in theatre settings/restricted and semi-restricted areas
- worn as PPE for procedures where splashing/spraying of body fluids is anticipated, and as source control when performing clean/aseptic procedures where risk of infection is deemed to be high
- well-fitting and completely cover the hair
- changed/disposed of at the end of a single clinical procedure/task or at the end of a theatre session (for sessional use): immediately if contaminated with blood and/or body fluids
- removed before leaving the theatre/clean room.
Resources
Further information can be found in the headwear literature review
For the recommended method of putting on and removing PPE see video below and Appendix 6.
The correct order for donning, doffing and disposal of PPE for healthcare workers from NHS National Services Scotland on Vimeo.
PPE for visitors
PPE may be offered to visitors to protect them from acquiring a transmissible infection.
If a visitor declines to wear PPE when it is offered then this should be respected and the visit must not be refused. PPE use by visitors cannot be enforced and there is no expectation that staff monitor PPE use amongst visitors. However, if staff or visitors identify the need for PPE, advice on its correct use should be provided by staff.
Visitors do not routinely require PPE unless they are providing direct care to the individual they are visiting.
The table below shows the PPE which should be worn where appropriate and when the visitor chooses to do so.
IPC Precaution |
Gloves |
Apron |
Face covering/mask |
Eye/Face Protection |
|---|---|---|---|---|
|
Standard Infection Control Precautions (SICPs) |
Not required*1 |
Not required*2 |
Where splash/spray to nose/mouth is anticipated during direct care |
Where splash/spray to eyes/face is anticipated during direct care |
|
Transmission Based Precautions (TBPs) |
Not required*1 |
Not required*2 |
If within 2 metres of service user with suspected or known respiratory infection |
If within 2 metres of service user with suspected or known respiratory infection |
*1 unless providing direct care which may expose the visitor to blood and/or body fluids i.e. toileting.
*2 unless providing care resulting in direct contact with the service user, their environment or blood and/or body fluid exposure i.e. toileting, bed bath.
1.5 Safe Management of Care Equipment

Care equipment is easily contaminated with blood, other body fluids, secretions, excretions and infectious agents. Consequently it is easy to transfer infectious agents from communal care equipment during care delivery.
Care equipment is classified as either:
- Single-use – equipment which is used once on a single patient and then discarded. Must never be reused even on the same patient. The packaging carries the symbol below.

- Needles and syringes are single use devices. They should never be used for more than one patient or reused to draw up additional medication.
- Never administer medications from a single-dose vial or intravenous (IV) bag to multiple patients.
- Single patient use – equipment which can be reused on the same patient.
- Reusable invasive equipment - used once then decontaminated for example surgical instruments.
- Reusable non-invasive equipment (often referred to as communal equipment) - reused on more than one patient following decontamination between each use e.g. commode, patient transfer trolley.
Before using any sterile equipment check that:
- the packaging is intact
- there are no obvious signs of packaging contamination
- the expiry date remains valid
Decontamination of reusable non-invasive care equipment must be undertaken:
- between each use
- after blood and/or body fluid contamination
- at regular predefined intervals as part of an equipment cleaning protocol
- before inspection, servicing or repair
Adhere to manufacturers’ guidance for use and decontamination of all care equipment.
All reusable non-invasive care equipment must be rinsed and dried following decontamination then stored clean and dry.
Decontamination protocols should include responsibility for, frequency of and method of environmental decontamination.
An equipment decontamination status certificate will be required if any item of equipment is being sent to a third-party, for example for inspection, servicing or repair.
Guidance may be required prior to procuring, trialling or lending any reusable non-invasive equipment.
Resources
Further information can be found in the management of care equipment literature review.
For how to decontaminate reusable non-invasive care equipment see Appendix 7.
1.6 Safe Management of Care Environment
 It is the responsibility of the person in charge to ensure that the care environment is safe for practice (this includes environmental cleanliness/maintenance). The person in charge must act if this is deficient.
It is the responsibility of the person in charge to ensure that the care environment is safe for practice (this includes environmental cleanliness/maintenance). The person in charge must act if this is deficient.
The care environment must be:
- visibly clean, free from non-essential items and equipment to facilitate effective cleaning
- well maintained and in a good state of repair
- routinely cleaned in accordance with the Health Facilities Scotland (HFS) National Cleaning Specification:
- a fresh solution of general-purpose neutral detergent in warm water is recommended for routine cleaning. This should be changed when dirty or at 15 minutes intervals or when changing tasks
- routine disinfection of the environment is not recommended. However, 1,000ppm available chlorine should be used routinely on sanitary fittings
- refillable bottles should not be used in settings where immunocompromised patients receive care (haematology and oncology, cardiac surgery, bone marrow and stem cell transplant, neonatal, paediatric and adult ICU, transplant units).
- where refillable bottles are appropriate for use, cleaning products should be freshly made (never topped up) and discarded after 24 hours (or sooner as dependent on manufacturers instructions). The refillable bottle should be washed and thoroughly dried between uses.
Staff groups should be aware of their environmental cleaning schedules and clear on their specific responsibilities.
Cleaning protocols should include responsibility for, frequency of and method of environmental decontamination.
When an organisation adopts decontamination processes not recommended in the NIPCM the care organisation is responsible for governance of and completion of local risk assessment(s) to ensure safe systems of work.
Resources
Further information can be found in the routine cleaning of the environment literature review.
1.7 Safe Management of Linen
 Clean linen
Clean linen
This is linen that has been processed (laundered) and is ready for use.
- Should be stored in a clean, designated area, preferably an enclosed cupboard.
- Clean linen should be stored separately from used and infectious linen.
- If clean linen is not stored in a cupboard then the trolley or pod used for storage must be designated for this purpose and completely covered with an impervious covering that is able to withstand decontamination.
- Hand hygiene should be performed before handling clean linen.
For all used or infectious linen
Used linen has been used by a non-infectious patient with no visible soiling or contamination by blood or body fluids.
Infectious linen has been used by a patient who is known or suspected to be infectious and/or linen that is contaminated with blood and/or other body fluids for example faeces.
- Any linen used during patient transfer, for example blankets, should be categorised at the point of destination.
- All linen that is deemed unfit for re-use, for example torn or heavily contaminated, should be categorised at the point of use and returned to the laundry for disposal.
When handling used or infectious linen:
- Refer to Appendix 15 for the selection of PPE required.
- Ensure a laundry receptacle is available as close as possible to the point of use for immediate linen deposit.
- Ensure linen is placed in the appropriate receptacle depending on categorisation.
- Check that linen is free from inappropriate items before placing into the laundry receptacle, for example used equipment/needles, service user personal belongings
- Place infectious linen directly into a water-soluble or alginate bag and secure, then place into a plastic bag, for example clear bag, and secure before placing in a laundry receptacle. This applies also to any items heavily soiled and unlikely to be fit for reuse.
- Used and infectious linen bags or receptacles must be tagged, for example: ward or care area and date.
- Perform hand hygiene as recommended in Section 1.2 after handling, bedmaking and bagging linen
Do not:
- rinse, shake or sort linen on removal from beds or trolleys
- place used linen on the floor or any other surfaces, for example a locker or table top
- re-handle used linen once bagged
- overfill laundry receptacles
Store all used or infectious linen in a designated, safe, lockable area whilst awaiting uplift. Uplift schedules must be acceptable to the care area and there should be no build-up of linen receptacles.
Service users, patients and their carers or relatives who are required to take clothing home to launder should be provided with the Washing Clothes at Home Leaflet
Local guidance regarding management of linen may be available.
Resources
Further information can be found in the safe management of linen literature review and National Guidance for Safe Management of Linen in NHSScotland Health and Care Environments - For laundry services/distribution.
Further information about linen bagging and tagging can be found in Appendix 8.
Scottish Government uniform, dress code and laundering policy is available.
1.8 Safe Management of Blood and Body Fluid Spillages
 Spillages of blood and other body fluids may transmit blood borne viruses.
Spillages of blood and other body fluids may transmit blood borne viruses.
Spillages must be decontaminated immediately by staff trained to undertake this safely.
Responsibilities for the decontamination of blood and body fluid spillages should be clear within each area/care setting.
If superabsorbent polymer gel granules for containment of bodily waste are used these should be used in line with national guidance. In Scotland refer to Safety Action Notice - SAN(SC)19/03 | National Services Scotland (nhs.scot)
Resources
For management of blood and body fluid spillages see Appendix 9.
Further information can be found in the management of blood and body fluid in health and care settings literature review.
1.9 Safe Disposal of Waste (including sharps)
 Scottish Health Technical Note (SHTN)03-01: NHSScotland Waste Management Guidance contains the regulatory waste management guidance for NHSScotland health and care services including waste classification, categorisation, segregation, storage, packaging, transport, treatment and disposal.
Scottish Health Technical Note (SHTN)03-01: NHSScotland Waste Management Guidance contains the regulatory waste management guidance for NHSScotland health and care services including waste classification, categorisation, segregation, storage, packaging, transport, treatment and disposal.
The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation to the safe disposal of sharps.
Waste regulations require the classification of waste based on hazardous characteristics.
Classification of waste
- Special (hazardous) waste. Special waste includes a range of controlled wastes, defined by legislation, which contain dangerous or hazardous substances. Examples of special (hazardous) waste resulting from healthcare activities includes sharps, infectious or potentially infectious clinical waste and some pharmaceuticals or medicinal wastes.
- Non-hazardous waste is residual waste produced in both clinical and non-clinical settings which may include dry recyclates (glass, paper and plastics, metals, cardboard), food waste, packaging waste and furniture.
Healthcare waste should be segregated at source into suitable colour-coded and appropriately labelled receptacles across all health and care settings in Scotland.
SHTN 03-01 contains a full colour-coded waste segregation guide which represents NHSScotland accepted best practice and ensures compliance with current regulations. The most frequently used waste streams in health and care settings are summarised below.
Waste streams
- Black (non-hazardous)
- Residual waste remaining after all source segregated recyclates have been removed.
- For treatment (which may include recovery of materials), then disposal.
- Orange (infectious), light blue (laboratory)
- Orange - consists of infectious or potentially infectious substances or items. Orange lidded leak resistant receptacles may be used for solidified infectious liquids and dialysis waste. Orange bags may be used for items such as PPE, spillage kits, swabs or dressings. Orange lidded sharps box used for sharps disposal only. Consigned to clinical waste treatment facility for treatment and disposal
- Light blue – laboratory/microbiological waste that must be autoclaved before disposal via the orange stream.
- Yellow, red (infectious)
- Yellow or red lidded leak resistant receptacles may be used for waste which poses ethical, highly infectious or contamination risks.
- This includes anatomical and human tissue which is recognisable as body parts but may include other types of waste that require incineration to comply with national or regional policy. Receptacles should be clearly labelled with contents.
- Yellow stream infectious waste is special (hazardous) waste.
- Consigned to clinical waste incineration facility for disposal (high temperature incineration).
Safe waste disposal at care area level
Always dispose of waste:
- immediately and as close to the point of use as possible
- into the correct segregated colour coded approved waste bag or container compliant with UN and relevant industry standards.
Liquid waste, (such as body fluids) that is not suitable for disposal via the toilet or macerator, must be rendered safe by adding a self-setting gel or compound before placing in a rigid leak-resistant receptacle.
Waste bags should not be overfilled and should be securely sealed when 3/4 full (manufacturer’s fill line for sharps boxes) using a closure technique such as a ‘swan neck’ to close with with a plastic tie or tape. The point of origin and date of closure must be clearly marked on the tape/tag or bag.
Store all waste in a designated, safe, lockable area whilst awaiting uplift. Uplift schedules must be acceptable to the care area and there should be no build-up of waste receptacles.
Sharps boxes should:
- have a dedicated handle
- have a temporary closure mechanism, which must be employed when the box is not in use
- be labelled with date of assembly, point of origin and date of closure.
- be disposed of when the manufacturers’ fill line is reached
Local guidance regarding management of waste at care level may be available.
Resources
Further information can be found in the safe disposal of waste literature review.
1.10 Occupational Safety: Prevention and Exposure Management (including sharps)
 Exposure in relation to blood borne viruses (BBV) is the focus within this section and reflects the existing evidence base.
Exposure in relation to blood borne viruses (BBV) is the focus within this section and reflects the existing evidence base.
The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation to:
- arrangements for the safe use and disposal of sharps
- provision of information and training to employees
- investigations and actions required in response to work related sharps injuries
Sharps handling must be assessed, kept to a minimum and eliminated if possible with the use of approved safety devices.
Manufacturers’ instructions for safe use and disposal must be followed.
Needles must not be re-sheathed/recapped.*
Always dispose of needles and syringes as 1 unit.
If a safety device is being used safety mechanisms must be deployed before disposal.
Occupational exposure
An occupational exposure is a percutaneous or mucocutaneous exposure to blood or other body fluids.
Occupational exposure risk can be reduced via application of other SICPs and TBPs outlined within the NIPCM.
Significant occupational exposure
A significant occupational exposure is a percutaneous or mucocutaneous exposure to blood or other body fluids from a source that is known, or found to be positive for a blood borne virus (BBV).
Examples of significant occupational exposures would be:
- a percutaneous injury, for example injuries from needles, instruments, bone fragments, or bites which break the skin
- exposure of broken skin, for example abrasions, cuts, eczema
- exposure of mucous membranes including the eye from splashing of blood or other high risk body fluids
There is a potential risk of transmission of a Blood Borne Virus (BBV) from a significant occupational exposure and staff must understand the actions they should take when a significant occupational exposure incident takes place. There is a legal requirement to report all sharps injuries and near misses to line managers/employers.
Additionally, employers are obligated to minimise or eliminate workplace risks where it is reasonably practicable. Immunisation against BBV should be available to all qualifying staff, and testing (and post exposure prophylaxis when applicable) offered after significant occupational exposure incidents.
Resources
For the management of occupational exposure incidents see Appendix 10
Exposure prone procedures (EPPs)
Exposure prone procedures (EPPs) are invasive procedures where there is a risk that injury to the healthcare worker may result in the exposure of the patient’s open tissues to the blood of the worker (bleed-back).
There are some exclusions for HCWs with known BBV infection when undertaking EPPs. The details of these and further information can be found in the occupational exposure management (including sharps) literature review.
* A local risk assessment is required if re-sheathing is undertaken using a safe technique for example anaesthetic administration in dentistry.
Content
- 1.1 Patient Placement/Assessment for infection risk
- 1.2 Hand Hygiene
- 1.3 Respiratory and Cough Hygiene
- 1.4 Personal Protective Equipment
- 1.5 Safe Management of Care Equipment
- 1.6 Safe Management of Care Environment
- 1.7 Safe Management of Linen
- 1.8 Safe Management of Blood and Body Fluid Spillages
- 1.9 Safe Disposal of Waste (including sharps)
- 1.10 Occupational Safety: Prevention and Exposure Management (including sharps)
References
- Reference 1
The use of the word 'Persons' can be used instead of ‘Patient’ when using this document in non-healthcare settings.